Mad Monk's Page
Of
Horn Dyeing
Click on picture to see a bigger one



Copper dyed horns
Mad Monk at Work
Iron dyed horns

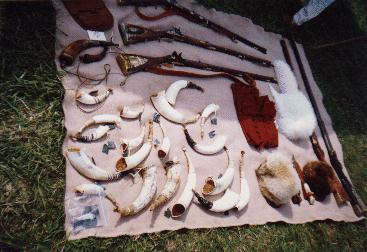
Horns at the 1994 Gunmaker's Fair
Horn Dyes
Historical.
Man has long used mineral pigment dyes
as a means of coloring
goods made from various animal proteins. In addition to being
decorative, the mineral pigment dyes also provide varying degrees
of protection against attack by insects or bacteria. The
mineral
pigment dyes are both decorative and protective.
While the mineral pigment dyes were used
on cellulose-based
goods, it was on protein-based goods where they were at their
greatest value. Animal proteins used by man include; leather,
fur, horns, silk, wool, bone and antler. Hides, leather,
horn,
silk and wool are all food sources for a wide number of animals
and microbes.
The use of certain mineral pigment dyes
is ancient in origin
and keep in mind that these were manufactured chemical substances.
About 2000 years ago, Pliny (The Elder), writing in 50 A.D. Rome,
described a method of testing the purity of verdigris (cupric
acetate) to determine if it had been adulterated with cheaper iron
salts (ferrous sulfate or copperas). Verdigris was vital
in the
preserving and tanning of leather. Pliny used a piece of
reed
that had been soaked in a solution of gallic or tannic acid and
then dried. A sample of verdigris was dissolved in water
and the
reed was then dipped into this solution. If the reed turned
black
the color change was proof of the presence of iron salts.
The reaction between gallic and tannic
acid with iron salts
was fairly well known since this formed the basis for black dyes
on protein-based goods.
Concept.
The mineral pigment dye is the acid salt
of a particular
metal. The metal, usually in a pure state, is reacted with
an
acid. Copper, iron, tin and lead were in common usage.
The
oldest process used vinegar or "sour wine" as a source of acetic
acid to form the acetate of the metal. Exactly when mineral
acids
came in use is unknown though Arabian manuscripts from 800 A.D.
give directions for the preparation of these acids. The mineral
acids being hydrochloric, sulfuric and nitric.
The protein-based goods were soaked, or
steeped, in a solu
tion of the mineral pigment dye in water. The dye solution
would
then permeate the protein. Protein has a high affinity for
acids
and the dyes would bind to the sulfur ions in the protein mole
cules.
After impregnating the protein with the
water-soluble
acid-metal salt it was necessary to convert the dye to a
water-insoluble pigment. This was usually done by "sweating"
the
goods after dyeing. In a water- insoluble form, the dye was
then
locked into the protein. Sweating consisted of hanging, or
placing, the goods in a chamber with steam. The acid-metal
salt
dyes will break down at temperatures of 150 degrees F on up to
212
degrees F. In the presence of air, the carbonate or oxide
form of
the metal results. This is where the term "mineral pigment
dye"
originates. Dyes are soluble in some type of a solvent.
Mineral
pigments are not soluble in a solvent.
Copper is an effective bacterial inhibitor.
To most insects
and worms copper is a potent toxin. By impregnating the protein
with certain metallic compounds the protein becomes toxic to most
life forms which would use it as a food source. Since the
metal
lic compound is insoluble and chemically bonded to the protein
it
will not be toxic to the user or wearer.
Until recently, the mineral pigment dyes
and mordant dyes
using a metallic compound were the only means available to protect
wool and silk against insect attack.
Mineral pigment dyes are noted for extreme
durability.
Sunlight has no effect on the final colors. Only acid wash
water
will leach the mineral pigment dyes from protein fabrics.
Protective versus decorative.
When used in small amounts, mineral pigment
dyes, such as
copper acetate, would be protective and not really decorative.
When used in larger amounts, the dye becomes both protective and
decorative.
Copper acetate, or verdigris, had been
widely used to
"pickle" protein-based goods. The term "pickle" being a process
whereby you use just enough copper to protect the item against
bacterial and insect attack. The term "green hides" referred
to
the actual color of animal hides prior to tanning. The term
"green horn" referred to the actual color of horns of commerce
prior to their being worked into a useful item.
Our ancestors, until recently, shipped
hides and horns
considerable distances. Shipment and storage was not always
under
the best of conditions. Pickling in a weak copper acetate
solu
tion insured that a hide would not sour nor would fly larvae eat
holes in the hide. After pickling, insect larvae will no
longer
eat the hide or the horn.
After a cattle horn has been boiled to
remove the bone core
it is somewhat pliable and its micro-pores are filled with water.
It is at this point where they were then placed in a solution of
copper acetate for several days. Water-soluble copper acetate
would then migrate into the water contained within the horn's
microscopic pores. The horns would then be removed from the
pickling bath and allowed to dry. Upon complete drying, horns
harden and "cure". While drying, water migrates to the surface
of
the horn carrying some copper acetate with it. When fully
dry,
the horns had a dark greenish colored crust on the surface.
Since
insect and bacterial attack would begin on the surface, the crust
insured that nothing of the sort would occur.
Decorative.
For decorative dyeing you would work the
horn down to its
final shape and size. All filing, scraping and abrasive smoothing
would be finished. The butt plug would have been made and
rough
fitted, but not fixed into the horn. The horn cannot be dyed
with
the butt plug in place.
The surface of the horn should be clean
and free of any
traces of skin oil.
Green horns.
By the 18th and 19th centuries there were
three copper dyes
in common usage on horn. Copper acetate (verdigris), copper
sulfate (bluestone, Roman vitriol) and nitrate of copper (cupric
nitrate).
For the shooter who just wants to do one
or two powder horns
I would suggest using copper sulfate. This is readily available
at
garden supply centers in one pound bags.
Cupric acetate is more difficult to obtain
and is considered
more dangerous to work with due to its toxicity if ingested.
For
someone who wants to do a number of horns and wants the exact
green coloration found in original powder horns, cupric acetate
is
a must.
Cupric nitrate was prepared by digesting
pure copper in
slightly diluted nitric acid. This is dangerous to prepare
and
use. This would be a must if very deep green, a bottle
green,
color is desired.
Preparation of the horn.
In attempting to dye a horn that has dried
and cured it will
be found that the horn will not accept anything more than a
surface dyeing. No matter how long you soak the horn in a
dye
bath you will gain little penetration of the horn by the dye.
It is suggested that the horn first be
boiled in clear water
for several hours. this will drive water into the microscopic
pores in the horn.
Horn is little more than a biological
modification of hair.
The horn has a pronounced fibrous structure and these fibers form
layers, or laminations, in the horn. Horn is nearly identical
to
human finger nails. The microscopic pores in a horn are actually
very minute spaces that occur along the bundles of hairs that make
up the structure and along minute gaps between the layers, or
laminations.
The mineral pigment dye solutions will
not tolerate boiling
water temperatures without breaking down. It is then best
to boil
the horn in water to saturate any available pore space with water.
When placed in the dye bath, the dye will then migrate into the
horn in areas that were filled with water by boiling in plain
water.
Using the term "decorative" in describing
one of the benefits
of dyeing horns is most apt. During the dyeing process, a
mineral
pigment is deposited along the horn's fibers and between layers
within the horn. After dyeing, the horn will show structural
detail patterns that were not visible prior to dyeing. Very
plain
horns show very noticeable structural details after dyeing.
No two horns are identical in structure
even when they came
off the same animal. Dyeing the horns only serves to enhance
this
individuality.
Dye baths.
To minimize the expense of dyeing just
a few horns, one would
want a dye bath container that holds the entire horn with a
minimum of free space around the horn.
Plastic jugs make durable dye baths.
Plastic containers used
to hold dog food, soaps, etc., make very good dye baths.
Look for
ones that are tall and narrow. At present, a Butcher's Blend
dog
food jug makes an ideal dye bath for horns up to 13 inches in
length.
Dye bath concentration.
It is difficult to give exact amounts
of dye "stock solu
tions" to be added to a dye bath simply because of the wide
variations in porosity exhibited by cattle horns. In the
same dye
bath, two horns may come out quite different in appearance due
to
differences in horn porosity.
Horns almost totally lacking in color
will generally dye
darker due to a greater porosity. Horns with a white body
will
pick up little dye in the white area since the white pigment, in
the horn, occupies void areas in the horn's microscopic structure.
Copper dyeing for green horns. When
using copper acetate,
dye concentration would be about 1/4 pound of copper acetate to
one gallon of water. When using copper sulfate, you would
add
copper sulfate to the water until no more will dissolve with
stirring.
In preparing a dye bath one should always
add several ounces
of vinegar to the bath as a "stabilizer". The acetic acid
in the
vinegar will help prevent the dye from converting to a
water-insoluble form during dyeing. The acetic acid in the
vinegar also assists in dye penetration during dyeing.
The dye bath should remain covered at
all times. Should the
dye bath have unlimited access to air, it will slowly show a
sediment on the bottom of the bath. The water-soluble metal
will
convert to the water- insoluble carbonate or oxide as oxygen or
carbon dioxide is adsorbed from the air in contact with the
surface of the liquid. This will be minimal with additions
of
vinegar in the dye solution.
Dyeing of the horn.
After the dye bath has been prepared,
it is simply a matter
of placing the previously boiled horn in the bath and then cover
ing the bath. How long to leave the horn in the bath is a
matter
of judgement. Several days should be considered minimum.
At the
same time, several weeks will not alter the amount of dye picked
up by the horn. A rule of thumb would be about one week.
Sweating
After dyeing, the horn should be "sweated"
to fix the dye in
the horn. In most cases the sweating is also required to
give the
proper color to the horn.
When the horn is removed from the dye
bath, most of the
mineral pigment dye within the horn's "pores" is still in a
water-soluble form. If the horn is simply allowed to air
dry,
most of the dye will migrate to the surface of the horn where
contact with air will result in the conversion of the dye to its
mineral pigment form, i.e., carbonate or oxide. The horn
is
"sweated" to give this conversion from water-soluble to
water-insoluble while the dye is still in the horn's micro-porous
structure. This process takes advantage of most mineral
pigment
dye's inability to withstand temperatures near the boiling point
of water. Heating the horn, in an atmosphere saturated
with
water, i.e., steam, results in the conversion of the water-soluble
dye to the water-insoluble pigment while the dye is still in the
horn rather than on the horn's surface.
How this sweating process is carried out
is sometimes criti
cal in determining the actual, and correct, color in the finished
horn.
Sweating chamber.
The sweating chamber is simply a large
metal bucket that is
covered while it is being heated. The dyed horn should be
placed
in the bucket in a position that prevents it from contacting the
sides of the bucket. Contact with the bucket, during steaming,
will result in spotting of the horn at the point, or points of
contact.
Some original horns which had been dyed
show these spots,
usually on the outside curve of the horn.
Take a piece of scrap 3/4" wood
and taper it to fit into the
large opening of the horn. Leave about 1 to 1 1/2" protruding
from the opening. This will keep the horn off the bottom
of the
bucket and keep the horn out of the small amount of water used
to
generate steam in the bucket. A piece of dowel in the spout
opening will hold the horn away from the sides of the bucket.
The horn is placed in the bucket with
enough water to cover
the bottom of the bucket. The bucket is then covered and
placed
on a heat source. Aluminum foil, two sections, will cover
most
buckets.
It is not necessary to boil the water
in the bottom of the
bucket. The idea being to keep the air in the bucket saturated
with water vapor while the temperature rises to about 200 degrees
F, or just below the boiling point of water.
Time.
The conversion of the dye, by sweating,
is rapid after the
temperature rises above 150 degrees F. Fifteen to thirty
minutes
being sufficient to fix the color within the horn.
The horn leaves the sweating pot somewhat
pliable. As soon
as the horn leaves the pot you would want to lightly press the
butt plug in place or in some manner insure the opening is round
upon cooling and drying.
Do not drive the plug in as the horn will
shrink as it looses
water. The horn picked up water during the dyeing and sweating
process. depending on the weather conditions it may take
from 2
days to a week to return to a dry condition. Drying produces
varying amounts of shrinkage, the exact amount depends on the
amount of water in the horn after dyeing.
Color variations.
If the horn is "dunged" after dyeing and
sweating it will
turn a color described as olive. The composting of animal
dung,
until recently, was the only source of a wide array of various
chemicals, some known as "manure salts". Dunging had been
a step
in the tanning of animal hides. Protein-based goods dyed
with
copper dyes were sometimes "dunged" to give a specific green
color. this color might best be described as olive, something
of
a brown-ish green color. Hydrogen sulfide, produced by bacterial
activity in the dung, changed a bottle green color to the brown-
ish green color.




